China conditionally approves four self-developed COVID-19 vaccines: official
Share - WeChat

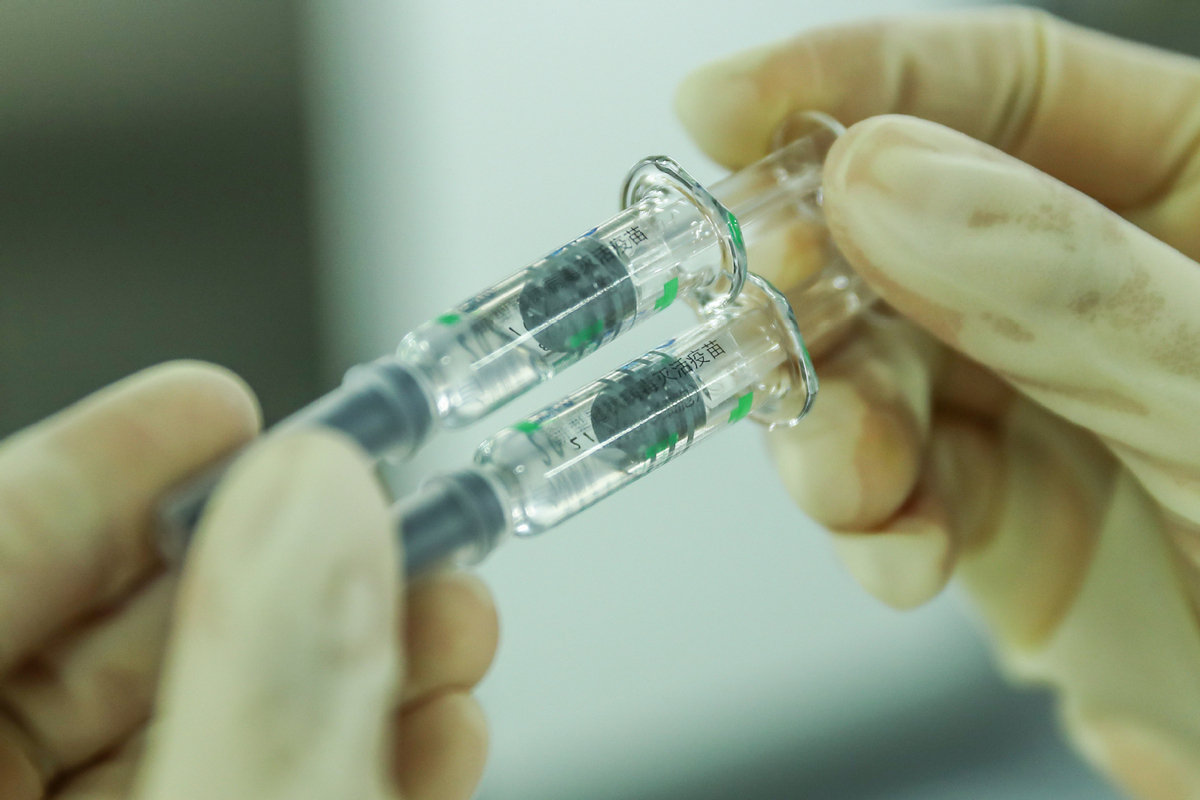
BEIJING - China has granted conditional market approval to four self-developed COVID-19 vaccines, Minister of Science and Technology Wang Zhigang said Friday.
China has adopted five technological approaches in developing COVID-19 vaccines. China now has 17 self-developed COVID-19 vaccines undergoing clinical trials, with seven undergoing phase-3 clinical trials, Wang told a press conference held in Beijing.
China has also included 11 drugs and treatment methods in its diagnosis and treatment plan for COVID-19, Wang added.
- Expert condemns DPP's obstruction of V-Day participation
- Student shuttle service launched between Hengqin and Macao
- Unbreakable lifeline: Gansu's sheepskin rafts powering China's WWII resistance
- Full text of Xi Jinping's speech at 25th Meeting of Council of Heads of State of SCO
- Conservation efforts revive Chishui River section in Zunyi
- Northeast Anti-Japanese United Army exhibit opens in Beijing